Leticia S. Sonon, David E. Kissel, and Uttam Saha
What are Cation Exchange and Cation Exchange Capacity?
Soil clay minerals and organic matter tend to be negatively charged, thus attracting positively charged ions (cations) on their surfaces by electrostatic forces. As a result, the cations remain within the soil root zone and are not easily lost through leaching. The adsorbed cations may easily exchange with other cations in the soil solution, hence the term "cation exchange." The adsorbed cations replenish the ions in the soil solution when concentrations decrease due to uptake by plant roots.
Cation exchange capacity (CEC) is a measure of the total negative charges within the soil that adsorb plant nutrient cations such as calcium (Ca2+), magnesium (Mg2+) and potassium (K+). As such, the CEC is a property of a soil that describes its capacity to supply nutrient cations to the soil solution for plant uptake. Figure 1 illustrates cations retained on soil clay minerals that can exchange with those in the soil solution. Plant roots can remove nutrients from the soil solution, which results in nutrients moving away from the clay particles. Addition of fertilizer to soil causes an initial increase in nutrient concentration in the soil solution, which results in nutrients moving toward clay particles.
The nutrient cations plants use in the largest amounts are potassium (K+), calcium (Ca2+) and magnesium (Mg2+). Other cations adsorbed on exchange sites are ammonium (NH4+), sodium (Na+), hydrogen (H+), aluminum (Al3+), iron (Fe2+ or Fe3+), manganese (Mn2+), copper (Cu2+) and zinc (Zn2+). Micronutrient cations such as zinc, copper, iron and manganese are typically present at very low concentrations in soils. Ammonium concentrations are also typically very low because microorganisms convert ammonium to nitrate in a process called nitrification.
How CEC changes with Soil pH
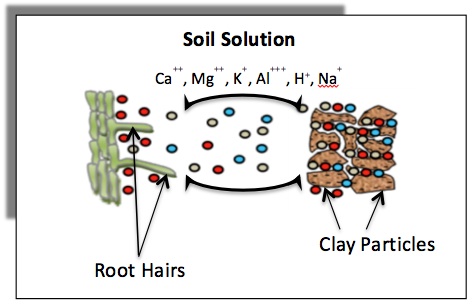 Figure 1.
Figure 1. Schematic diagram showing exchange of cations between the soil surfaces and the soil solution, and the movement of these cations from soil solution to roots (rhizosphere) for uptake.
The CEC of soil organic matter and some clay minerals varies with pH. Generally, the CEC is lowest at soil pHs of 3.5 to 4.0 and increases as the pH is increased by liming an acid soil, as shown in Figure 2. Because CEC may vary considerably with soil pH, it is a common practice to measure a soil's CEC at a pH of 7.0. Also note that some positive charges may occur on specific soil mineral surfaces at low pH. These positive charges retain anions (negatively charged ions) such as chloride (Cl-) and sulfate (SO42-).
Calculating the Cation Exchange Capacity from a Routine Soil Test
The CEC value included on typical soil testing laboratory reports is calculated by adding together the concentrations (expressed as milliequivalents of charge per 100 grams of soil) of potassium, magnesium, calcium, sodium and hydrogen, which are extracted from soils using an appropriate extraction method. 海角官方首页 Soil Testing Laboratory uses the Mehlich I procedure, based on a double acid (0.05 N HCl + 0.025 N H2SO4) extracting solution. This method is appropriate for acidic, low CEC soils, which are commonly found in Georgia. The CEC of soils containing large amounts of clay or organic matter, or that are alkaline, cannot be satisfactorily analyzed using the Mehlich I extract. Other soil extraction methods should be used on these types of soils.
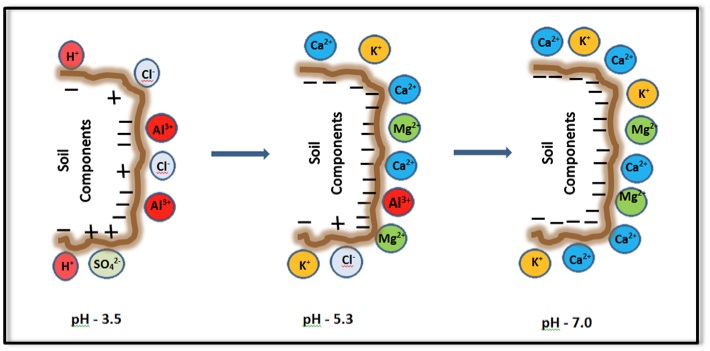 Figure 2.
Figure 2. Influence of pH on the surface charge of soil and its components.
Typical CEC Values in Soils
In most soil reports, CEC is expressed as milliequivalents (meq) of charge (number of charges) per 100 grams of soil (meq/100 g or as cmol/kg when using International Scientific Units). The number of milliequivalents is used rather than a weight (pounds, grams, etc.) of adsorbed cations because CEC represents the total number of charges, which is a better standard of comparison of different soils because each cation species has a different weight and soils differ in the proportions of the different cation species.
Table 1 shows the typical cation exchange capacities of soil clay minerals and soils of various textures. Because soil is a mixture of different particle sizes (sand, silt and clay), clay mineral types and organic matter in various proportions, the dominant components and soil pH dictates the soil?s CEC.
| Table 1. Cation exchange capacities at pH 7.0 of different soil types, textures and soil organic matter. |
| Soil and Soil Components |
CEC (meq/100 g) |
| Clay Type |
|
| Kaolinite |
3-15 |
| Illite |
15-40 |
| Montmorillonite |
80-100 |
| Soil Texture |